Whether a Reaction Is Exothermic or Endothermic Is Determined by
As illustrated in Figure 114 the formation of a solution may be viewed as a stepwise process in which energy is consumed to overcome solute-solute and solvent-solvent attractions endothermic processes and released when solute-solvent attractions are established an exothermic process referred to as solvation. For an endothermic reaction heat is absorbed making the net enthalpy change positive.

Heat Of Reaction Reflects The Difference In Enthalpy Between The Products And The Reactants Teaching Chemistry Chemistry Education Teaching Science
This can be done by preheating the input.

. The total energy involved in this conversion is equal to the experimentally determined enthalpy of formation textΔH_textf of the compound from its elements. What is the approximate amount of heat involved in the dissolution assuming the heat capacity of the resulting solution is 418 Jg C. In this case the overall change is exothermic.
Van t Hoff plot for an exothermic reaction. Depending on the industrial process this fact can lead to trouble because energy must be provided to the system. If the energy required to break bonds is more than the.
If the energy required to break bonds is less than the energy released when forming new bonds the reaction will be exothermic. Hesss law can also be used. The relative magnitudes of the energy changes.
There are two sides to any chemical reaction. Commercial instant cold packs typically use either ammonium nitrate or urea as their salt component. Calculations of this type will also tell us whether a reaction is exothermic or endothermic.
When a chemical reaction occurs the balance between these two processes determines if the reaction is endothermic or exothermic overall. Le Chateliers principle states that a change in temperature pressure or concentration of reactants in an equilibrated system will stimulate a response that partially off. Figure 511 In a calorimetric determination either a an exothermic.
It is possible to predict whether a reaction will be endothermic or exothermic by doing a little math. Thus hydrogen bonds predominantly form because of the enthalpic factor. At which the reaction occurs and whether the reaction occurs under conditions of constant pressure or constant volume.
What two factors favor a spontaneous reaction. This shifts chemical equilibria toward the products or reactants which can be determined by studying the reaction and deciding whether it is endothermic or exothermic. When the salt is dissolved in water the ionic bonds of.
An exothermic reaction ΔH. When an endothermic reaction occurs the heat required is absorbed from the thermal energy of the solution which decreases its temperature Figure 511. For this it helps to know a bit about chemical reactions and chemical bonds.
A reaction must absorb energy equal to the enthalpy of the reaction to occur. Assume for the following situations that Beers law remains linear. Chemical reactions can be either exothermic give out heat or endothermic take in heat.
Therefore it is an endothermic reaction. Is the reaction exothermic or endothermic. However others disagree and believe that the entropic and.
A reaction will be endothermic if breaking the old bonds takes more enthalpy than the enthalpy released when the new bonds form. As the salt disassociates heat is either released in an exothermic reaction or absorbed in an endothermic reaction. By knowing the change in heat it can be determined whether or not a reaction is exothermic releases heat or endothermic absorbs heat.
Enter the email address you signed up with and well email you a reset link. The reactants are always on the. Total reduction with hydrogen is endothermic.
Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. It will be exothermic if the new bonds release more enthalpy than the enthalpy needed to break the old. However if more energy is needed to break the bonds than the energy being released energy is taken up.
How it does this and whether it favours the reactants or the products will depend on the reaction. A positive change in entropy and a negative change in enthalpy are the two contributors towards spontaneity. The law states that the total enthalpy change during a reaction is the same whether the reaction is made in one step or in several steps.
Prior to substitution into the Gibbs free energy equation the entropy change is converted to kJKmol. Is the reaction spontaneous at 25 C. Reduction with carbon monoxide is exothermic.
Hydrogen bonds are exothermic 3 and the heat derives from the energy released with the formation of the chemical bonds of the product. These reactions happen in a similar manner. In contrast hydrophobic bonds are endothermic and probably driven by the entropic factor 3.
On one side are the reactants. This means that for reduction with hydrogen energy must be added to the system to guarantee a constant reduction temperature. A reactant is the substance or substances that you start with.
Whether a process can occur spontaneously depends not only on the enthalpy change but also on the entropy change S and absolute temperature T. When the reaction is endothermic Δ r H 0 and the gas constant R 0 so Thus for an endothermic reaction the Van t Hoff plot should always have a negative slope. Identify whether the process is exothermic or endothermic.
Remember me on this computer. The purpose of the experiment was to investigate the enthalpy changes in several reaction and relationship of three exothermic reaction with hesslaw. Hot packs often use magnesium sulfate or calcium chloride.
The amount of heat absorbed or released under this condition is the enthalpy change H for the reaction where H. On the other side are the products. Thus according to the definition of the slope.
For a physical process and for a chemical reaction explain how heat is transferred released or absorbed at the molecular level. For a and b indicate whether the extinction coefficient ε path length b or concentration C is changing and how it will change the absorbance of the solution. Calorimetry also plays a large part of everyday life controlling the metabolic rates in humans and.
Dissolving 30 g of CaCl 2 s in 1500 g of water in a calorimeter Figure 2 at 224 C causes the temperature to rise to 258 C. This is known as an exothermic reaction. Close Log In.
A A sample is moved from a test tube that is 10 cm wide to a test tube that is 15 cm wide. A The spontaneity is determined by the entropy and the enthalpy. Log in with Facebook Log in with Google.
Identify what gains heat and what loses heat in a calorimetry experiment. It can be determined whether a reaction is endothermic or exothermic from it. The temperature change along with the specific heat and mass of the solution can then be used to calculate the amount of heat involved in either case.
In other words if a chemical change takes place by several different routes the overall enthalpy change is the same regardless of the route by which the chemical change occurs provided the initial and final condition are the same. In the laboratory many reactions are conveniently carried out at constant pressure in beakers or flasks that are open to the atmosphere.

Physical Chemistry Sign Of Enthalpy For Exothermic And Endothermic Reactions Chemistry Stack Exchange

Endothermic Reaction Definition Equation Graph Examples

Edumission Chemistry Form 5 Chapter 4 Exothermic And Endothermic Reaction Chemistry Lessons Chemistry Classroom Science Chemistry

For This Endothermic And Exothermic Reactions Lab Students Will Perform Two Chemical Reactions And M Exothermic Reaction Teaching Chemistry Chemical Reactions

Exothermic Vs Endothermic Chemistry S Give And Take Discovery Express
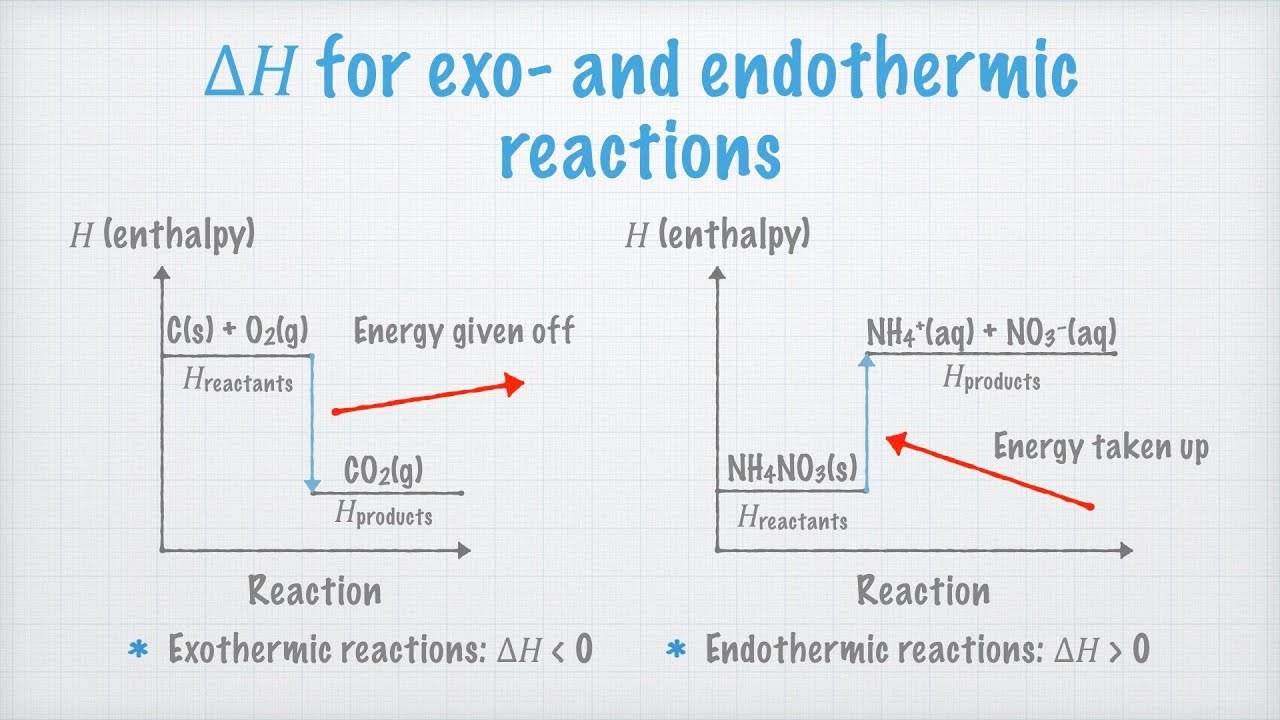
Endothermic And Exothermic Reactions Enthalpy Youtube

Exothermic Reactions Release Energy Endothermic Reactions Consume Energy Exothermic Reaction Homeschool Science Chemistry
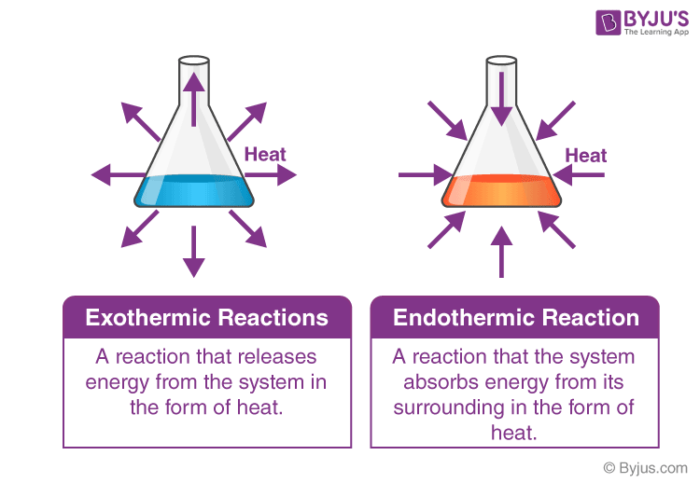
Difference Between Endothermic And Exothermic Reactions Chemistry

Potential Energy Diagram Practice Endothermic And Exothermic Reactions Potential Energy Energy Activities Exothermic Reaction

H Is A State Function Because E P V Are State Functions So It Depends Only On The Difference Betwee Chemistry Education Teaching Chemistry Science Chemistry

The Chemistry Website Will Allow Students Access To Notes Clear Instructions Review Materials And S Teaching Chemistry Chemistry Classroom Chemistry Lessons

Endothermic Vs Exothermic Reaction Graphs Youtube

Endothermic Exothermic Reactions Energy Changes In Chemical Reactions Mcat Content

Activation Energy Endothermic And Exothermic Reactions Via Youtube Energy Activities Exothermic Reaction Science Chemistry

Endothermic And Exothermic Reactions Lab Iteachly Com

Endothermic Vs Exothermic Reactions Chemtalk

Endothermic Vs Exothermic Reactions Chemtalk

Image Result For Exothermic Reaction Exothermic Reaction Chemistry Teaching Chemistry

Comments
Post a Comment